


The ingredient (called "NFD"with superior work for human health maintenance) was detected from
tree tea “Taheebo NFD” which natural vitality generated.
And many people drink the tea habitually since its release for 30 years.
“TAHEEBO NFD”is the tea made from the tree's inner bark.
The tree that serves as the basic ingredient for comes only from the Tabebuia avellanedae species in the Bignoniaceae family, a tree that is indigenous to the specific region of the Amazon basin in South America.
“TAHEEBO NFD”contains a lot of various nourishment ingredients in a good balance, including
vitamins and the mineral which are essential to health.
Bcause the tree tea doesn't contains an additive and caffeine at all,anyone
can drink with ease.
We deliver “Taheebo NFD” to all the ountries of the world
Theebo is a mysterious tree that is indigenous to the region of the Amazon basin in South America.
It has been drinking for 1500 years.It is an attractive tree as it was
drunk as a tea in the Inca Empire.




Turn up bast and identify it as Taheebo

Taheebo felled with a chain saw

Taheebo is hard and heavy than imagined.
The chain saw is not always useful.

felled stump of the Taheebo tree.
This is around 30 years old.

Bast of Taheebo

Taheebo found in a Brazilian botanical garden.Because the trees do not crowd like the jungle, there are many twigs.The appearance of the tree is considerably different from the one grows up in the jungle.

Young tree of Taheebo growing wild in the jungle

It is estimated to be a young tree aged 3 of Taheebo



Of today’s numerous so-called “health teas”, most are artificially cultivated or synthesized.
The reasoning behind this approach is that pure, natural ingredients are only available in very small quantities, limiting their usefulness as basic ingredients. Moreover, harvesting such substances requires restraint in order to protect these natural resources, and it’s difficult to ensure consistent quality in terms of their composition.
Despite these challenges,“TAHEEBO NFD”is produced using only bast (inner bark) from extremely rare wild trees.Although it may seem that the price of“TAHEEBO NFD”is high, considering preservation and the quality side of limited natural resources, it is unavoidable.

Tabebuia avellanedae, the tree from which“TAHEEBO NFD”is produced

The Amazon River

Dr. Accorsi holding bark from Tabebuia avellanedae

Just 7 millimeters of inner bark between the outer bark and woody tissue is used as a basic ingredient.

Inner bark chips, the basic ingredient for“TAHEEBO NFD”(carefully selected for their“NFD”content).
The wood that serves as the basic ingredient for “TAHEEBO NFD” comes only from the Tabebuia avellanedae species in the Bignoniaceae family (scientific name: Bignoniaceae Tabebuia avellanedae Lor. ex. Gris), a tree that is indigenous to the forests of the Amazon basin in South America.
There are at least 100 species belonging to the genus Tabebuia throughout
the Americas.Tabebuia avellanedae, a tree with a single purplish red flowering
species that is indigenous to a specific region of the Amazon basin in
South America becomes the material wood of “TAHEEBO NFD”.
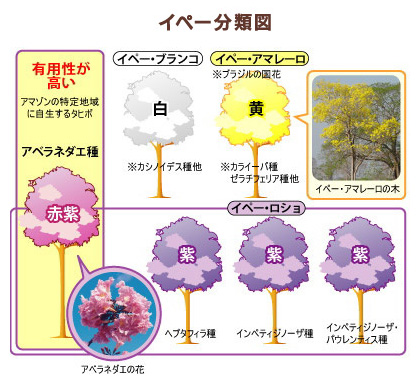

Purplish-red flowers of the species avellanedae

Tabebuia avellanedae as seen from above the jungle canopy
We have not made the location of the harvesting region public in order
to protect the valuable natural resources found there from indiscriminate
and illegal logging.
Instead, the company refers to the area as the“specific region”.
Deep in the forests of the Amazon basin is the region from which TAHEEBO
JAPAN harvests trees.
Some 70% of the Tabebuia avellanedae trees that grow there can be harvested
as raw material for “TAHEEBO NFD” .

We select only high-quality trees that meet our standards for “NFD” content for use as raw material for “Taheebo NFD.” . Samples are tested beforehand to assess “NFD” content.

Since taheebo is very hard and heavy tree, even if we use a chain saw, we can cut down only two trees per day.

Harvested trees are transported by truck to our manufacturing facility.(It takes about 1week.)

The bark is stripped from the trees at the manufacturing facility (at this point, the inner bark is still attached to the outer bark).

The bark is dried in natural sunlight (In a process that takes about 3months).

Dried bark is transported by truck to our local factory in Brazil.(It takes about 10days.)

The bark is dried again after arriving at the local factory.(It takes about 2months.)

At this point, the inner bark has separated naturally from the outer bark.
Inner bark that has separated from the outer bark is brushed and cut into small pieces.
The cut inner bark is further cut into small chips of 2 ~ 3 cm each by machine.

The chips are processed with live bacteria and dried again in natural sunlight (in a process that takes about 2 months). Dried chips are packed into 20 kg hemp sacks.

The chips are dried inside the hemp sacks as they await shipment in a warehouse (in a process that takes about 6months).
The raw material chips are transported by container ship to our Japanese warehouse(in a process that takes about 1month).
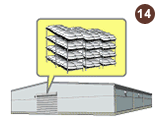
Raw materials chips are stored at our warehouse after arrival in Japan.
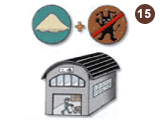
Chips are crushed, powderized, and sterilized at a subcontractor's facility that meets food product safety standards.

A final quality test is performed, and the product is complete.
And we can send you“TAHEEBO NFD”completed this quality inspection.
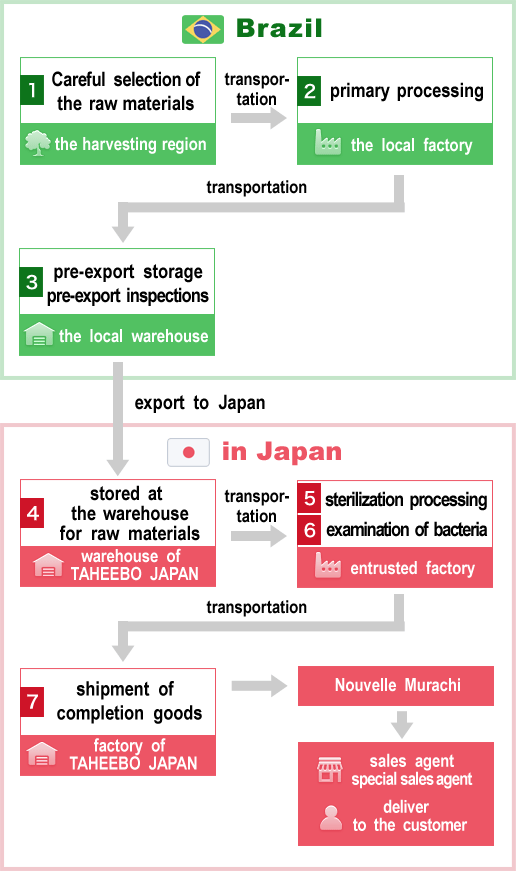
Taheebo(botanical name;Tabebuia avellanedae) tree - the raw materials of"Taheebo NFD"- is not a cultivated trees but a tree which is indigenous to the forests of the Amazon basin in South America.So it's very valuable and the choice of the raw wood to log is an important point.
Raw woods - the raw materials of"Taheebo NFD"-are harvested from the specific region※1 based on the survey by specialized team knowing a lot about South America plant.Besides, by prior sampling analysis,we systematically fell only the raw wood aged 30 years or more and contains the useful ingredient "NFD"reaches the standard value of TAHEEBO JAPAN (manufacturer)in its bark sample.
※1 We have not made the location of the harvesting region public in order to protect the valuable natural resources found there from indiscriminate and illegal logging. Instead, the company refers to the area as the "specific region".

TAHEEBO JAPAN (manufacturer) does not leave the securing of raw materials to a local company.We have a branch in Brazil Sao Paulo City,and we let a Japanese local representative be resident.The local representative is familiar with environment around the Amazon and local circumstances.
The bark is stripped from the felled trees at the manufacturing facility, and is transported to a local factory In Brazil.The bark is dried in natural sunlight.Naturally separated bast(inner bark) is further cut into small chips of 2 〜 3cm each.

The local factory In Brazil

Chips are packed into hemp sacks.And They are transported to a local storage warehouse.

finely cut chips of inner bark
The chips are dried inside the hemp sacks in a local storage warehouse (about 6months).
During this period, we perform quality control analysis※2 before the export to Japan.
※2 Viewing and microscope confirmation, purity, chemical composition, useful ingredient measurement, confirmation of the microbe (based on quality guidelines RDC204・RDC210 of Federative Republic of Brazil hygiene supervision agency

The situation in the local storage warehouse

Quality control analysis before the export to Japan

Quality control analysis before the export to Japan
The raw materials tip imported by Brazil is carefully controlled in the warehouse of the manufacturer(TAHEEBO JAPAN)※3 with the automatic ventilating device to maintain good state of preservation.
※3 Location of TAHEEBO JAPAN's (manufacturer's) warehouse for raw materials is not announced publicly to protect them from theft risks.

warehouse of TAHEEBO JAPAN,Co,Ltd.(the manufacturer)in Japan
Processing from raw materials tip to powder, processing from essence extraction into essence powder, and processing to a soft capsule are carried out in the outer entrusted factory of TAHEEBO JAPAN which adapted to food safety standards (GMP standard)

The storage condition in TAHEEBO JAPAN's (manufacturer's) warehouse for raw materials
In each factory, we carry out the quality check based on the administrative guidelines including the examination of bacteria on product at any time.
In addition, we carry out the strict examination by the the third party organization of inspection regularly, and the test result report is submitted to the manufacturer(TAHEEBO JAPAN) each time.
Inspection of processing from raw materials tip into powder
In addition to an examination of sterilization processing and bacteria, TAHEEBO JAPAN perform 340 items of pesticide residue inspection based on the Positive List System.
Because Taheebo is not the cultivation crops, it is not originally possible that a pesticide is detected, but is to be concerned about the present conditions that environmental pollution scatters on a global scale.

powder made from crushed raw materials tip
Inspection at the time of the extract powder product making
In the process from essence extraction to spray-dry treatment to make extract powder, we carry out PH inspection, bacteria inspection, water check.

TAHEEBO ESSENCE Inspection at the time of the extract powder product making(Japan)
Inspection at the time of the soft capsule product making
We carry out an examination of bacteria, an examination by the viewing for appearance, size and the weight check of every grain by the computer, bottle weight check by the viewing.
TAHEEBO JAPAN(the manufacturer) pursue safety about other raw materials to add at the time of processing by requesting to submit the specification of each and every item.

TAHEEBO NAFDIN Filling process to soft capsules(Japan)

Inspection of TAHEEBO NAFDIN capsules(Japan)
The safety of the packing material has been confirmed.
About a desiccating agent bundled with packing material and a product, we carry out analysis and evaluation of composition and the influence on contents from the standpoint of harmfulness and stability to confirm safety of the material to use.
The delivered half-finished goods from entrust factory are given final check in the factory of TAHEEBO JAPAN,Co,Ltd.. And every product is wrapped by each package and it waits for shipment.
In addition, we make shrink packaging on each package to enhance safety (prevention of damage and contamination) at the distribution stage.

inspection and ackaging operation in the factory of TAHEEBO JAPAN(the manufacturer)

All the completion products ship from a direct management factory in Japan.
We have the system to route retrieval of the product by the lot number of manufactured product, the raw material lot number, the date of manufacture, the time of shipping and the destination.

Engraving the lot number
Today, a variety of healthy foods are seen in the market while the users increase year by year. Meanwhile, many products doubtful in terms of their safety are sold among them in fact. Foods can have effects on the health of people by intake, and the safety is more critical issue than anything else. Based on the recognition that safety for human health is essential, TAHEEBO JAPAN requested independent and objective testing laboratories to conduct a range of safety studies. The study results have confirmed the safety of “TAHEEBO NFD” as a food.
試験会社: Study laboratories:Japan SLC, Inc. (Contract Research Department) / BML, Inc. (Safety Evaluation Department)
| Acute oral toxicity study | |
| Study name | Single dose oral toxicity study of Avellanedae extract powder in rats |
|---|---|
| Study article | Avellanedae extract powder |
| Study objective | A dose of Avellanedae extract powder is administered orally in male and female rats, and the acute toxicity is examined. |
| Study result | No death was observed in both male and female groups administered with the study article. The minimum lethal dose exceeded 2000 mg/kg. The changes of body weight were similar to those of the control group. The autopsy of all the specimens revealed no abnormality. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
| Mutagenicity study(Ames test) | |
| Study name | Mutagenicity study of Avellanedae extract powder in microorganisms |
|---|---|
| Study article | Avellanedae extract powder (BML-2606) |
| Study objective | Avellanedae extract powder is tested to determine whether it has a mutagenic effect induced by microorganisms. |
| Study result | Avellanedae extract powder is considered to have no mutagenic effect induced by any strains of Salmonella typhimurium (TA100, TA1535, TA98, and TA1537) and Escherichia coli (WP2 uvrA), regardless of whether it is in active metabolic state or not. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
BML, Inc. (Safety Evaluation Department) Study no.: 4329
| Mutagenicity study(Ames test)-2 | |
| Study name | Mutagenicity study of Avellanedae extract powder in microorganisms (with adjuvant) |
|---|---|
| Study article | Avellanedae extract powder (BML-4708) |
| Study objective | Avellanedae extract powder is tested to determine whether it has a mutagenic effect induced by microorganisms. |
| Study result | Avellanedae extract powder is considered to have no mutagenic effect. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
BML, Inc. (Safety Evaluation Department) Study no.: 6640
| Chromosome aberration test | |
| Study name | Chromosome aberration test of Avellanedae extract powder in mammalian cell culture |
|---|---|
| Study article | Avellanedae extract powder (BML-3757) |
| Study objective | Avellanedae extract powder is tested to determine whether it has clastogenic property in mammalian cell culture (CHL/IU cells). |
| Study result | This study was performed based on a pilot test. Any results of the direct method or the metabolic activation method of the study did not indicate a property of Avellanedae extract powder to induce structural or numeric chromosome aberrations. In conclusion, the study article is considered to have no clastogenic property in Chinese hamster lung fibroblast cell line (CHL/IU). |
Study laboratory:Japan SLC, Inc. (Contract Research Department)
BML, Inc. (Safety Study Division, Cell Biology Department) Study no.: 5618
| Eye irritation Avellanedae extract powder in rabbits | |
| Study objective | Avellanedae extract powder is administered in the eyes of young mature rabbits to determine its primary irritation property on eye mucosa, and the results are reported. |
|---|---|
| Study result | The solution of Avellanedae extract powder dissolved in 0.05% physiological saline is considered to have no irritation property on eye mucosa of rabbits. |
Study laboratory:Japan SLC, Inc. (Contract Research Department)
| Primary skin irritation test of Avellanedae extract powder in rabbits | |
| Study objective | Avellanedae extract powder is applied on the rabbit skin to determine its primary irritation property on skin, and the results are reported. |
|---|---|
| Study result | The solution of Avellanedae extract powder dissolved in 0.1% acetone was classified as a mild irritant. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
| Skin sensitivity study of Avellanedae extract powder in guinea pigs | |
| Study objective | Avellanedae extract powder is tested to determine its skin sensitivity in guinea pigs, and the results are reported. |
|---|---|
| Study result | Avellanedae extract powder is considered to have no noticeable level of skin sensitivity in guinea pigs. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
| Phototoxicity test of Avellanedae extract powder in guinea pigs | |
| Study objective | Avellanedae extract powder is tested to determine its phototoxicity on the skin of guinea pigs, and the results are reported. |
|---|---|
| Study result | Avellanedae extract powder is considered to have no noticeable level of phototoxicity on the skin of guinea pigs. |
Study laboratory: Japan SLC, Inc. (Contract Research Department)
| Photosensitization test of Avellanedae extract powder in guinea pigs | |
| Study objective | Avellanedae extract powder is tested to determine its photosensitizing property in guinea pigs by the Adjuvant and strip method, and the results are reported. |
|---|---|
| Study result | No positive skin reaction was observed in any of the animals photosensitized and photochallenged with Avellanedae extract powder. Avellanedae extract powder is considered to have no photosensitizing property. |
Study laboratory:Japan SLC, Inc. (Contract Research Department)
| Long-term intake test | |
| Study name | Study of Avellanedae tea containing indigestible dextrin for the effect on the reduction of blood sugar increase and the safety after long-term intake. |
|---|---|
| Study article | Avellanedae tea containing indigestible dextrin |
| Study objective | Avellanedae extract powder containing indigestible dextrin is tested to determine the safety in human after the long-term intake. |
| Study result | Twelve healthy volunteers took Avellanedae extract powder containing indigestible dextrin 3 times a day for 12 weeks, and the physical, blood pressure, and laboratory measurements were performed subsequently. No significant change was observed in the measurements, and the long-term continuous intake did not indicate any clinically relevant problem. |
Study laboratory:The article on this study was presented in Journal of
Nutritional Food (2001) vol. 4, no.4.
We deliver “Taheebo NFD” to all the ountries of the world
Nouvelle Murachi Co.,Ltd.
1437-1 Nishikawa Ryuo-cho Gamo-gun Shiga Pref
520-2572 Japan.
TEL +81-748-58-2377